Kotlikoff Lab
The Kotlikoff laboratory focuses on (a) the molecular processes underlying cardiac and smooth muscle cell development, function and dysfunction and (b) the development and application of optical tools for in vivo imaging at the molecular level, under the auspices of CHROMusTM.
 Molecular Processes Underlying Cardiac Development and Repair:
Molecular Processes Underlying Cardiac Development and Repair:
The Kotlikoff laboratory first identified the presence of c-kit positive cells in the neonatal heart and their unique developmental potential at that stage, and contrasted that potential with adult c-kit positive cells. At the early post-natal stage, these cells undergo myodifferentiation and heart repair. Following this period of post-natal heart development, c-kit positive cells adopt only vascular lineages and drive neovascularization. The laboratory has shown definitively that these cells are endogenous to the adult heart (do not arise from circulating endothelial cell precursors) and cannot adopt cardiac fates. By contrast, neonatal precursors are not committed to cardiac fates, but undifferentiated c-kit+ cells from the neonate are capable of adopting such fates in vitro, in explants to scid mice, and when transplanted to adult hearts.
- Tallini, Y, KS Greene, M Craven, A Spealman, M Hesse, J Smith, A Woods, B Singh, A Yen, B Fleischman, and MI Kotlikoff. c-kit+ precursors in the neonatal heart specify all cardiac lineages. Proc Natl Acad Sci 106:1808-13, 2009. PMC2644119
- Jesty, SA, MA Steffey, FK Lee, M Breitbach, M Hesse, S Reining, JC Lee, RM Doran, AY Nikitin, BK Fleischmann, and MI Kotlikoff. Cardiovascular precursors support postinfarction myogenesis in the neonatal, but not the adult, heart. Proc Natl Acad Sci. USA 109:13380-13385, 2012. PMC3421216
CHROMusTM: The development and application of optical tools for in vivo imaging at molecular scale:
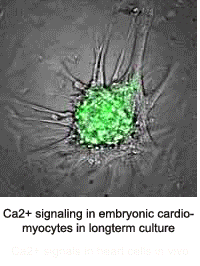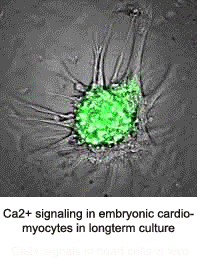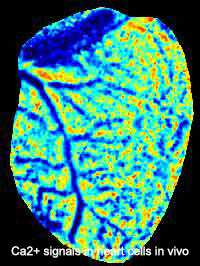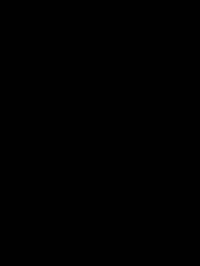
The Kotlikoff lab published the first use of genetically encoded sensors for in vivo measurements in mice. This development was made possible by collaboration with the laboratory of Junichi Nakai and an improvement of the original GFP-based Ca2+ sensor, GCaMP. That sensor misfolded at body temperature and could only be used for ex-vivo measurements at room temperature. Multiple stabilizing mutations led to the development of GCaMP2 and the exciting demonstration of lineage specific cardiac imaging in mice in vivo, in real time, demonstrating the use of this system to understand embryonic heart development. The Kotlikoff lab, in collaboration with the lab of Holger Sondermann, subsequently determined the basis of conditional fluorescence of GCaMP molecules, which relies on the rapid, Ca2+ -dependent protonation and de-protonation of the chromophore.
CHROMusTM(http://chromus.vet.cornell.edu/), under the direction of Michael Kotlikoff, is an NIH funded resource committed to developing fifty transgenic mouse lines designed for combinatorial crosses enabling the co-expression of sensors and rhodopsin based effectors, or red and green Ca2+ sensors in interacting lineages. We use novel combinations of sensors, in vivo imaging preparations, and lineage -targeted BAC transgenic mice and distribute these lines around the country and world, allowing researchers to lead the discovery of novel physiology processes in the heart, brain, lung and immune systems. The resource enables researchers to conduct experiments not otherwise possible in a cost effective and highly efficient manner, facilitating the use of optical genetic tools and markedly accelerating scientific discovery.
- Shui B, Lee JC, Reining S, Lee FK, Kotlikoff MI. Optogenetic sensors and effectors: CHROMus-the Cornell Heart Lung Blood Institute Resource for Optogenetic Mouse Signaling. Front Physiol. 2014 Nov 6;5:428. PMID 25414670
- Tallini, NY, M Ohkura, B-R Choi, G Ji, K Imoto, R Doran, J Lee, P Plan, J Wilson, H-B Xin, A Sanbe, J Gulick, J Robbins, G Salama, J Nakai, and MI Kotlikoff. Imaging Cellular Signals in the Heart in Vivo: Cardiac expression of the high signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci 103:4753-4758, 2006. PMID: 16537386.
- Wang, Q, B Shui, MI Kotlikoff, H Sondermann. Structural basis for calcium sensing by GCaMP2. Structure 16:1817-1827, 2008. PMC2614139
Kolikoff Research in the news:
- Cornell Chronicle: Glowing hearts
- Nature Editorial on arrhythmia paper
- Cornell Chronicle article on Nature paper
- Optogenetic toolkits: CHROMus


