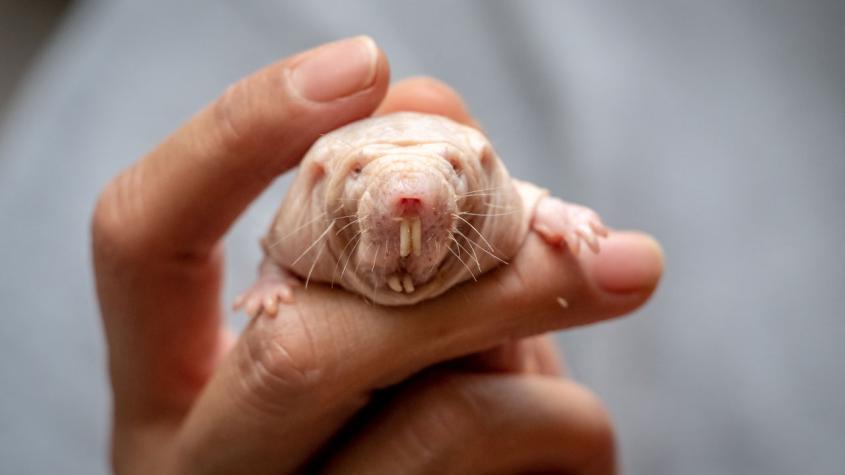
Study unlocks clues in mystery of naked mole-rats’ exceptional fertility
Unlike humans and other mammals, which become less fertile with age, naked mole-rats can reproduce throughout their remarkably long lifespans. A new study, published today in Nature Communications, sheds light on unique processes that bestow the rodents with what seems like eternal fertility, findings that could eventually point to new therapies for people.
“Naked mole-rats are the weirdest mammals,” said lead author Dr. Miguel Brieño-Enríquez, assistant professor at Magee-Womens Research Institute and the University of Pittsburgh School of Medicine’s Department of Obstetrics, Gynecology and Reproductive Sciences. “They’re the longest-lived rodent, they almost never get cancer, they don’t feel pain like other mammals, they live in underground colonies, and only the queen can have babies. But to me, the most amazing thing is that they never stop having babies — they don’t have a drop in fertility as they age. We want to understand how they do this.”
For most mammals, including humans and mice, females are born with a finite number of egg cells, which are produced in utero via a process called oogenesis. Because this limited supply of egg cells depletes over time — some are released during ovulation, but most simply die — fertility declines with age.
In contrast, naked mole-rat queens can breed right through old age, suggesting the rodents have special processes to preserve their ovarian reserve and avoid waning fertility.
“There are three possibilities for how they do this: They are born with a lot of egg cells, not as many of these cells die, or they continue to create more egg cells after birth,” said Brieño-Enríquez. “My favorite hypothesis is that they use a cocktail of all three.”
Sure enough, Brieño-Enríquez and his team found evidence for each of the three processes.
The researchers compared ovaries from naked mole-rats and mice across different stages of development. Despite their similar sizes, mice live four years at most and start to show a drop in fertility by nine months, whereas naked mole-rats have a life expectancy of 30 years or more.
They found that naked mole-rat females have exceptionally large numbers of egg cells compared to mice and that death rates of these cells were lower than in mice. For example, at 8 days old, naked mole-rat females had 1.5 million egg cells per ovary, about 95 times more than mice of the same age.
Most remarkably, the study found that oogenesis happens postnatally in naked mole-rats. Egg precursor cells were actively dividing in 3-month-old animals, and these precursors were found in 10-year-old animals, suggesting that oogenesis could continue throughout their lives.
“This finding is extraordinary,” said senior author Dr. Ned Place, professor at the Cornell University College of Veterinary Medicine. “It challenges the dogma that was established nearly 70 years ago, which stated female mammals are endowed with a finite number of eggs before or shortly after birth, without any additions being made to the ovarian reserve thereafter.”
Naked mole-rats live in colonies of several dozen to hundreds of individuals. Like bees or ants, colony members divvy up tasks, including providing defense, digging tunnels, caring for young and collecting food. Only the single dominant female in a colony can breed, and she suppresses reproduction in other females to maintain her queenly status.
“Unlike bees or ants, a female naked mole-rat is not born a queen,” explained Brieño-Enríquez. “When the queen dies or is removed from the colony, a subordinate female becomes reproductively activated and takes her place. Any girl can become a queen.”
To learn more about this process, the researchers removed 3-year-old females from the colony to prompt reproductive activation and compared these new queens with subordinate females. They found that non-breeding subordinates had egg precursor cells in their ovaries, but the cells started dividing only after a transition to queen.
“This is important because if we can figure out how they’re able to do this, we might be able to develop new drug targets or techniques to help human health,” said Brieño-Enríquez. “Even though humans are living longer, menopause still happens at the same age. We hope to use what we are learning from the naked mole-rat to protect ovary function later in life and prolong fertility.”
“But the ovary is more than just a baby factory,” he continued. “Ovary health influences cancer risk, heart health and even lifespan. Better understanding of the ovary could help us find ways to improve overall health.”
Other authors who contributed to the study were Mariela Faykoo-Martinez, Dr. Michael D. Wilson and Dr. Melissa M. Holmes, of the University of Toronto; Meagan Goben, Patrick T. Walsh and Dr. Samia H. Lopa, of the University of Pittsburgh; Dr. Jennifer K. Grenier, Ashley McGrath, Dr. Alexandra M. Prado, Jacob Sinopoli, Kate Wagner and Dr. Paula E. Cohen, of Cornell; and Dr. Diana J. Laird, of the University of California San Francisco.
This research was funded by the National Institutes of Health (R00HD090289, P50HD096723 and P50HD076210), the Natural Sciences and Engineering Research Council of Canada (RGPIN2011-402633, RGPIN 2018-04780 and RGPAS 2018-522465), the Ontario Early Researcher Award, the W.M. Keck Foundation Award, the Empire State Stem Cell Fund (C30293GG) and the Magee-Auxiliary Woman Scholar endowment.
Learn more about the Magee-Womens Research Institute and the University of Pittsburgh School of Medicine.
Written by Asher Jones, University of Pittsburgh